ABSTRACT
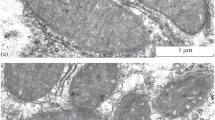
In the present work, ultrastructural changes of rat liver mitochondria in hyperthyroidism were studied. Hyperthyroidism was induced in male Wistar rats by daily administration of 100 μg thyroxin per 100 g body weight for 5 days. The level of triiodothyronine and thyroxine increased 3- and 4-fold, respectively, in comparison with the same parameters in the control group, indicating the development of hyperthyroidism in experimental animals. It was found that under this experimental pathology 58% of the mitochondria are swollen, with their matrix enlightened, as compared to the control. In 40% of the profiles, the swollen mitochondria in the liver under hyperthyroidism exhibited rounded mono- or multilayer membrane structures, called lamellar bodies (LBs), presumably at different stages of their development: from the formation to the release from the organelles. Most LBs were located in the mitochondria near the nuclear zone (27%), while their number was reduced in the part of the cell adjacent to the plasma membrane. In a number of swollen mitochondria the cristae were shown to change their orientation, being directed radially toward the center of the mitochondria. We suggested that it is the first stage of formation of LBs. The second stage can be attributed to the formation of monomembrane structures in the center of the organelles. The third stage is characterized by the fact that the membrane of the lamellar bodies consists of several layers, and in this case the bodies were located closer to the outer mitochondrial membrane. The evagination of the outer mitochondrial membrane and its connection with lamellar structure can be recognized as the fourth stage of formation of LBs. At the fifth stage the developed lamellar formations exited the mitochondria. At the same time, following the exit of LBs from the mitochondria, no damage to the mitochondrial membrane was registered, and the structure of the remaining part of the mitochondria was similar to the control. The nucleus of the hepatocyte also underwent structural changes in hyperthyroidism, exhibiting changes in the membrane configuration, and chromatin condensation. The nature and structure of the LBs, as well as their functional role in the liver mitochondria in hyperthyroidism, require further investigation.







Similar content being viewed by others
Abbreviations
- LBs:
-
lamellar bodies
REFERENCES
Carter WJ, Shakir KM, Hodges S, Faas FH, Wynn JO (1975) Effect of thyroid hormone on metabolic adaptation to fasting. Metabolism 24(10):1177–1183
Fan GC, Kranias EG (2011) Small heat shock protein 20 (HspB6) in cardiac hypertrophy and failure. J Mol Cell Cardiol 51(4):574–577
Ferreira PJ, L'Abbate C, Abrahamsohn PA, Gouveia CA, Moriscot AS (2003) Temporal and topographic ultrastructural alterations of rat heart myofibrils caused by thyroid hormone. Microsc Res Tech 62(5):451–459
Gainutdinov MK, Konov VV, Ishmukhamedov RN, Zakharova TN, Khalilova MA, Mamatova ZA, Asrarov MI, Mirmakhmudova SI (1990) Regulation of thyroid hormones of the interaction of mitochondria with low-molecular weight cytoplasmic mediators, induced by phosphate- dependent transport of K+ and H+ ions through the mitochondrial inner membrane. Biokhimiia 55(12):2239–2246 [Russian]
Goglia F, Moreno M, Lanni A (1999) Action of thyroid hormones at the cellular level: the mitochondrial target. FEBS Lett 452(3):115–120
Haagsman HP, Van Golde LM (1991) Synthesis and assembly of lung surfactant. Annu Rev Physiol 53:441–464
Hackenbrock CR (1968) Ultrastructural bases for metabolically linked mechanical activity in mitochondria. II. Electron transport-linked ultrastructural transformations in mitochondria. J Cell Biol 37(2):345–369
Kononenko V, Erman A, Petan T, Križaj I, Kralj S, Makovec D, Drobne D (2017) Harmful at non-cytotoxic concentrations: SiO2-SPIONs affect surfactant metabolism and lamellar body biogenesis in A549 human alveolar epithelial cells. Nanotoxicology 11(3):419–429
Wai T, Langer T (2016) Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrinol Metab 27(2):105–117. https://doi.org/10.1016/j.tem.2015.12.001
Videla LA (2000) Energy metabolism, thyroid calorigenesis, and oxidative stress: functional and cytotoxic consequences. Redox Rep 5:265–275. https://doi.org/10.1179/135100000101535807
Maity S, Kar D, De K, Chander V, Bandyopadhyay A (2013) Hyperthyroidism causes cardiac dysfunction by mitochondrial impairment and energy depletion. J Endocrinol 217(2):215–228
Mannella CA, Pfeiffer DR, Bradshaw PC, Moraru II, Slepchenko B, Loew LM, Hsieh CE, Buttle K, Marko M (2001) Topology of the mitochondrial inner membrane: dynamics and bioenergetic implications. IUBMB Life 52(3–5):93–100
Oosterlaken-Dijksterhuis MA, van Eijk M, van Buel BL, Van Golde LM, Haagsman HP (1991) Surfactant protein composition of lamellar bodies isolated from rat lung. Biochem J 274:115–119
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17(1):208–212
Quesada A, Sainz J, Wangensteen R, Rodriguez-Gomez I, Vargas F (2002) Osuna A. Nitric oxide synthase activity in hyperthyroid and hypothyroid rats. Eur J Endocrinol 147(1):117–122
Shears SB, Bronk JR (1980) Ion transport in liver mitochondria from normal and thyroxine-treated rats. J Bioenerg Biomembr 12(5–6):379–393
Soboll S (1993) Thyroid hormone action on mitochondrial energy transfer. Biochem Biophys Acta 1144(1):1–16
Acknowledgements
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Venediktova, N.I., Pavlik, L.L., Belosludtseva, N.V. et al. Formation of lamellar bodies in rat liver mitochondria in hyperthyroidism. J Bioenerg Biomembr 50, 289–295 (2018). https://doi.org/10.1007/s10863-018-9758-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-018-9758-8